Engineering E. coli to produce an enzyme that breaks down plastics
According to Miami researchers, the enzyme can break down plastic to its original chemical building blocks.
Engineering E. coli to produce an enzyme that breaks down plastics
Jason Boock and Jason Berberich, both professors of Chemical, Paper, and Biomedical Engineering at Miami University, are researching methods to produce an enzyme that has large potential to change how plastics are recycled and broken down.
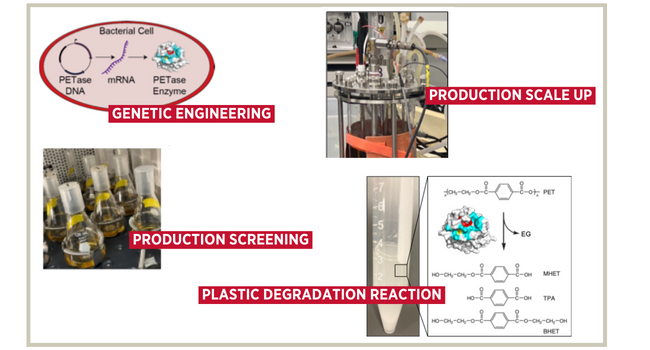
Professors Boock and Berberich are working with a team of graduate and undergraduate Miami University engineering students to develop an engineered E. coli bacterium that produces an enzyme which can break down plastic to its original chemical building blocks (monomers). They’re doing this by introducing DNA into the E. coli, which encodes for the genes for an enzyme. Through this process, the bacterial cells are programmed to “make the mRNA and then the enzyme that we're looking to make,” said Professor Boock.
Why has E. coli been chosen for this enzyme creation? “We use it because we know how to manipulate it really easily,” said Professor Boock. “It's incredibly well studied. We know its genetics. It grows very fast, and it grows on inexpensive food sources.” “E. coli is typically thought of as this virulent organism that makes you sick,” said Professor Boock. However, “the one that we work with in the lab is actually completely safe. It has the lowest biosafety hazard level.”
Once the enzyme has been produced within the bacteria, the research team purifies the enzyme through a process called chromatography. “The challenge is we have to get our protein away from all the rest of the proteins,” said Professor Boock. “We've engineered our enzyme to stick to a resin, while none of the cell’s other proteins will. And then we can have the enzyme unbind, resulting in a pure product.” Once the enzyme is extracted, it is placed in a tube with plastic particles and shaken. Then, the plastic begins breaking down into individual monomers for recycling.
Limits of “traditional” recycling
According to Professor Boock, there are many different ways to go through the plastics recycling process, each with its own benefits and downsides. With traditional recycling, Professor Boock said, “you can just mechanically break down the plastic into smaller chunks. While this is a significantly less expensive process than using enzymes, it doesn’t break down the plastic into its original materials – it just breaks them up into smaller pieces.” Plastics recycled in this way have limitations, says Tim Cameron, Ph. D., professor of mechanical engineering and member of Miami University’s Sustainability Committee. “Engineered plastic parts can only use about two percent recycled materials without compromising their strength or durability. What makes Professor Boock and Professor Berberich’s work so pivotal is that it breaks down polymers to individual monomers that are the building blocks for like-new (virgin) plastics.”
Because breaking down plastics to their original monomers via E. coli-produced enzymes is an expensive process, Professors Boock and Berberich and their team of student researchers (Chris MacFarlane, Luke Carter, Sam Karlock, and Anthony Greene) are currently investigating ways to make the process more efficient. “We're focused, at least right now, on trying to get the cells to make as much enzyme as possible,” said Professor Boock. “We've been playing tricks with manipulating the cells’ genetic makeup, so that it makes the protein that we want to in as high of an amount as possible. And then we have a student who's taken that from the benchtop scale to a bioreactor so that we can increase the amount that we're making.” By making this process cheaper, it starts to open up possibilities for commercial or industrial use at scale.
“We’ve seen a dramatic increase in production”
Through the research of Professor Boock, Professor Berberich and their student team, progress is being made. In their quest to increase the amount of enzymes produced by E.coli through genetic engineering, they’ve seen a 10-fold enhancement and production. Through their work with a bioreactor, they’ve seen another five-fold production increase on top of that. All told, that’s “50-fold more than what most people were getting in the past,” said Professor Boock. “We've seen a dramatic increase in production.”
With this increase in production comes new opportunities in the field. “I think we've developed a really good strategy to make this enzyme for other people, including us, to study it or do a lot more with it,” said Professor Boock.