
New study shows inflammation signals can induce complete retina regeneration in the embryonic chick
A new study by Miami University developmental biologist Katia Del Rio-Tsonis and colleagues reveals a previously unsuspected, new function of complement components that has a positive effect on eye tissue repair – including retina regeneration.
Del Rio-Tsonis and colleagues from Miami, the University of Pennsylvania and the University of Dayton have recently published a study that shows inflammation signals can induce complete retina regeneration in the embryonic chick, which is a powerful model to study neural regeneration.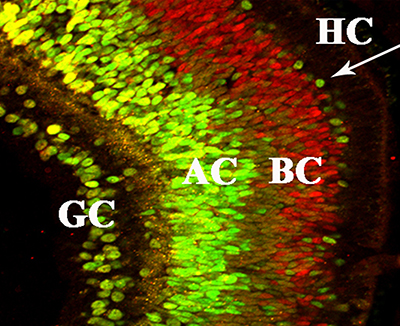
Chick retina regenerated by complement component C3 is nicely laminated and contains all major neural retina cell types. (Image courtesy Katia Del Rio-Tsonis)
Identifying the initiation signals for tissue regeneration in vertebrates is one of the major challenges in regenerative biology, say the study authors.
Their paper was published in the Aug. 14 issue of Nature Communications.
"There are several retina degenerative diseases such as age-related macular degeneration (AMD), retinitis pigmentosa, diabetic retinopathy and even glaucoma where retinal neurons die, causing reduced vision or blindness," Del Rio Tsonis explained. "Once those retina cells die, they are not replaced and therefore regenerative therapies are of the essence."
Repair versus disease
"The complement system is a surveillance system in our body that guards against disease and infections and coordinates the immune response with other programs associated with immune cells," Del Rio-Tsonis said.
"It is a first line of defense against foreign invaders and upon recognition of pathogens is activated and programmed to tag, destroy and eliminate."
During injury and disease in mammals, inflammation signals are also produced. "It is critical to know which of those signals, as well as how much of those signals, are conducive for repair/regeneration versus scarring or further damage," Del Rio-Tsonis explained.
Mechanism for retina regeneration based on injury and inflammation signals
The researchers show that complement fragment C3a is sufficient to induce complete regeneration of the embryonic chick retina from stem/progenitor cells present in the eye.
Their results establish a mechanism for retina regeneration based on injury and inflammation signals – opening new avenues of experimentation in the field.
The embryonic chick eye provides a unique – and powerful – model to test factors and drugs for their potential to induce nerve cell regeneration and to dissect the molecular mechanisms involved in the process, according to the researchers.
Understanding of these mechanisms can lead to further testing in other vertebrate models, including mammals, which lack the ability to induce tissue regeneration.
Potential therapies for retina degenerative diseases
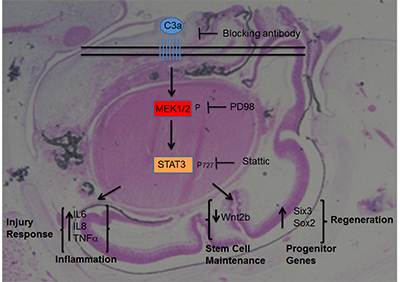
Histological representation of an embryonic chick eye undergoing retina regeneration. The eye was collected seven days after complete retina removal and addition of complement component C3a (image courtesy Katia Del Rio-Tsonis).
The main goal of regenerative medicine is to be able to induce tissue regeneration. The use of animal models, such as the embryonic chick, that have the ability to regenerate is critical in the quest to find regenerative therapeutics."A clear understanding of the mechanisms by which C3a induces retina repair and regeneration will help determine when and how much complement components are necessary to allow for repair versus disease," say the study authors.
Study authors
The study was a collaboration among scientists from Miami's department of biology and Center for Visual Sciences at Miami University (CVSMU); Panagiotis Tsonis, professor of biology and director of the University of Dayton's Center for Tissue Regeneration and Engineering at Dayton (TREND); and John Lambris, the Dr. Ralph and Sallie Weaver Professor of Research Medicine, and other scientists in the department of pathology and laboratory medicine at the University of Pennsylvania. Lead author was Tracy Haynes, former postdoctoral researcher and current senior lecturer at Miami.
Written by Susan Meikle, university news and communications, meiklesb@MiamiOH.edu
