
Miami microbiologists' research on biofilms uncovers new clues about how to treat pneumonia
By Susan Meikle, university news and communications, meiklesb@MiamiOH.edu.
During infection many types of bacteria organize into naturally occurring three-dimensional structures called biofilms. Bacterial biofilms cause chronic infections, because they show much greater resistance to both antibiotics and the immune system than their free-living counterparts.
Biofilms allow the bacteria to adhere to a variety of surfaces, living or nonliving. Some bacteria form biofilm infections of medical devices such as catheters and mechanical ventilators; once the device is colonized, infection is almost impossible to eliminate.
There are currently no approved treatments for biofilm-related infections: Though any bacterial infection can be treated with antibiotics, as of yet there are no specific methods to make them more effective against biofilms.
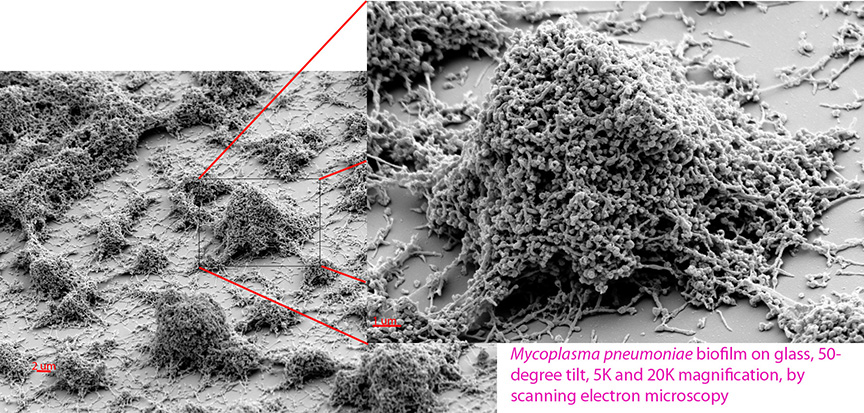
Microbiologist Mitchell Balish, associate professor of microbiology at Miami University, and his graduate students are working to understand the features of Mycoplasma pneumoniae biofilms and determine how their development might be inhibited.
A recent study by Monica Feng and Steven Distelhorst, graduate students in Balish's laboratory, sheds light on how biofilms of the bacterium M. pneumoniae organize themselves.
Their study also highlights the anti-biofilm potential of a group of chemical compounds called 2-aminoimidazoles (2-AIs) that could lead to improved treatment of patients.
Mycoplasma pneumoniae

Mycoplasma pneumoniae cells. Arrows indicate attachment organelles on two of the cells (image by Mitchell Balish).
Mycoplasma pneumoniae is a prevalent bacterium that is a leading cause of bronchitis and pneumonia, especially in children and young adults. It is also linked to asthma, autoimmune diseases and central nervous system infections.
It infects millions around the world on an annual basis and is responsible for more than 100,000 hospitalizations per year in the United States alone, Feng said.
In the last 20 years M. pneumoniae has gone from being entirely susceptible to ordinary antibiotics to being almost completely antibiotic resistant in populations in certain parts of the world, particularly East Asia, Feng said.
In the face of rising antibiotic resistance, development of new treatments for mycoplasmal diseases will rely on a deeper understanding of the basic biology of these unusual organisms, according to Balish.
Characterizing biofilms of M. pneumoniae and understanding how the bacterium interacts with human tissues will contribute to the development of new treatments for pneumonia and other diseases.
Characterizing M. pneumoniae: biofilm basics
Biofilms of M. pneumoniae — a simplified organism that contains only about 700 genes — have not been thoroughly characterized.
Feng and Distelhorst conducted a study to investigate the organization and developmental timeline of M. pneumoniae biofilms.
Using scanning electron microscopy at 5,000 and 20,000 magnification, the researchers found that "despite their genetic simplicity, these bacteria undergo significant changes in appearance during biofilm maturation," Feng said.
The bacterial biofilms were also treated with a 2-aminoimidazole (2-AI) compound — an emerging class of small molecules, derived from marine sponges, that have the ability to disrupt biofilms formed by many bacteria.
Results showed that the 2-AI compound interfered with growth of M. pneumoniae biofilms, "even though this simplified organism lacks any of the known molecular targets of 2-AIs," Feng said.

Follow the Balish Lab on Facebook at www.facebook.com/BalishLab.
This work not only establishes some of the basics about M. pneumoniae biofilms "but also paves the way for the creation of new treatments for patients infected with this organism," Feng said. "It may reveal more information about 2-AIs and their potential for combating a wide variety of bacterial infections around the globe."
Feng, who led the study, received a $500 travel award from the American Society for Microbiology (ASM) to present a poster of her work at the General Meeting of the ASM in New Orleans, May 30-June 2.
Feng's poster was selected as an Outstanding Student Poster by the ASM, an honor "dedicated to highlighting exceptional students for outstanding research efforts."
The 2-AI compound was synthesized by Christian Melander, Howard Schaeffer Distinguished Professor of Chemistry, at North Carolina State University. Its use in the Balish lab was facilitated by John Cavanagh, William Neal Reynolds Professor of Biochemistry, also at NCSU.
